C. elegans microinjection¶
Contributed by Ian Chin-Sang, Queens University, ON, Canada
One way to make transgenic animals in C. elegans we use a microinjection technique. Briefly, a DNA construct (plasmid, cosmid or YAC) or PCR product with your gene(s) of interest is mixed with a co-injection marker and injected into the distal gonad (syncytium). The injected DNA is taken up into the mature oocyte’s nucleus. The DNA exists as an extrachromosomal array (i.e. not integrated in the chromosome) which segregates randomly and can be lost, that is why we need a marker to follow which animals have the array. F1 progeny that show the co-injection marker phenotype are picked and transgenic lines are established by keeping those animals that segregate the array in their F2 generation. Usually 1 in 10 F1 progeny with the array will give you a transgenic line.
Typical injection concentration:
- 1-30 ng/ul Test plasmid
- 30 ng/ul co-injection marker (common markers pRF4 (roller) or p
Use pBluescript (or other plasmid) to bring total concentration of DNA to 100 ng/ul.
We do not use injection buffer T.E./E.B. or water seems to work fine.
Method¶
- Finding the correct spot to inject: A common problem for novice injectors is not aiming for the center of the gonad. To find the middle of the gonad arm you need to focus so that there are two rows of nuclei on each side of the gonad (see figure below) this ensures that you are at the core of the gonad.
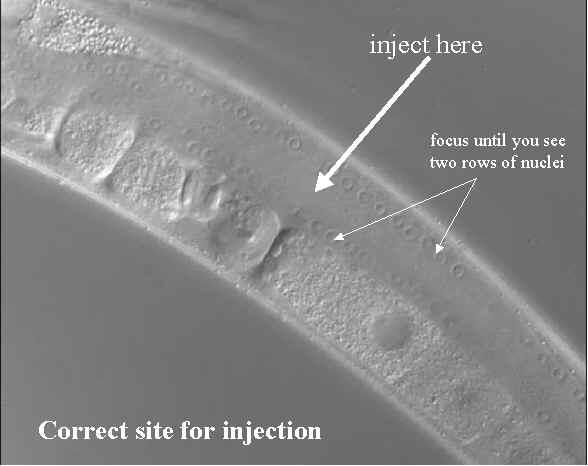
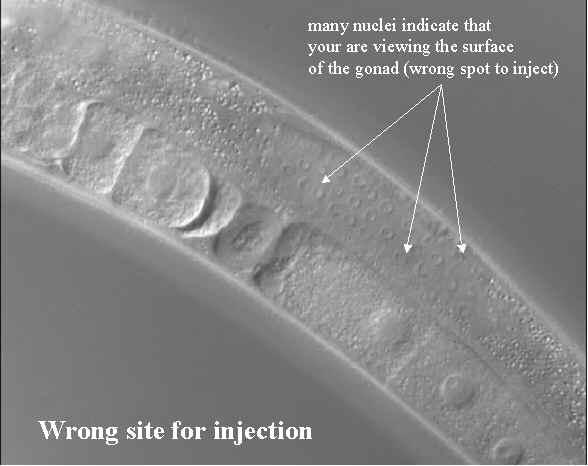
- If you see plenty of nuclei you are at the surface of the gonad and when you try and inject you will certainly miss.
This method is based, with permission, on an original protocol available here.